39 ndc 27808-065-02
NDC 27808-065 Oral Solution Promethazine Hydrochloride and Codeine ... Promethazine Hydrochloride and Codeine Phosphate is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Tris Pharma Inc. The primary component is Promethazine Hydrochloride; Codeine Phosphate. Packaging NDC 27808-065-02 473 mL in 1 BOTTLE, PLASTIC (27808-065-02) NDC SPL Data Element Entries 27808-065 Ndc - Promethazine Hydrochloride and Codeine Phosphate 27808-065-02: 473 mL in 1 BOTTLE, PLASTIC (27808-065-02) ML: NDC Record. Field Name Field Value Definition; PRODUCT NDC: 27808-065: The labeler code and product code segments of the National Drug Code number, separated by a hyphen. Asterisks are no longer used or included within the product code segment to indicate certain configurations of the ...
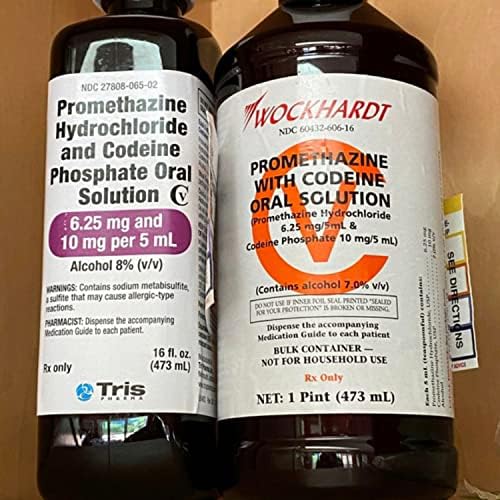
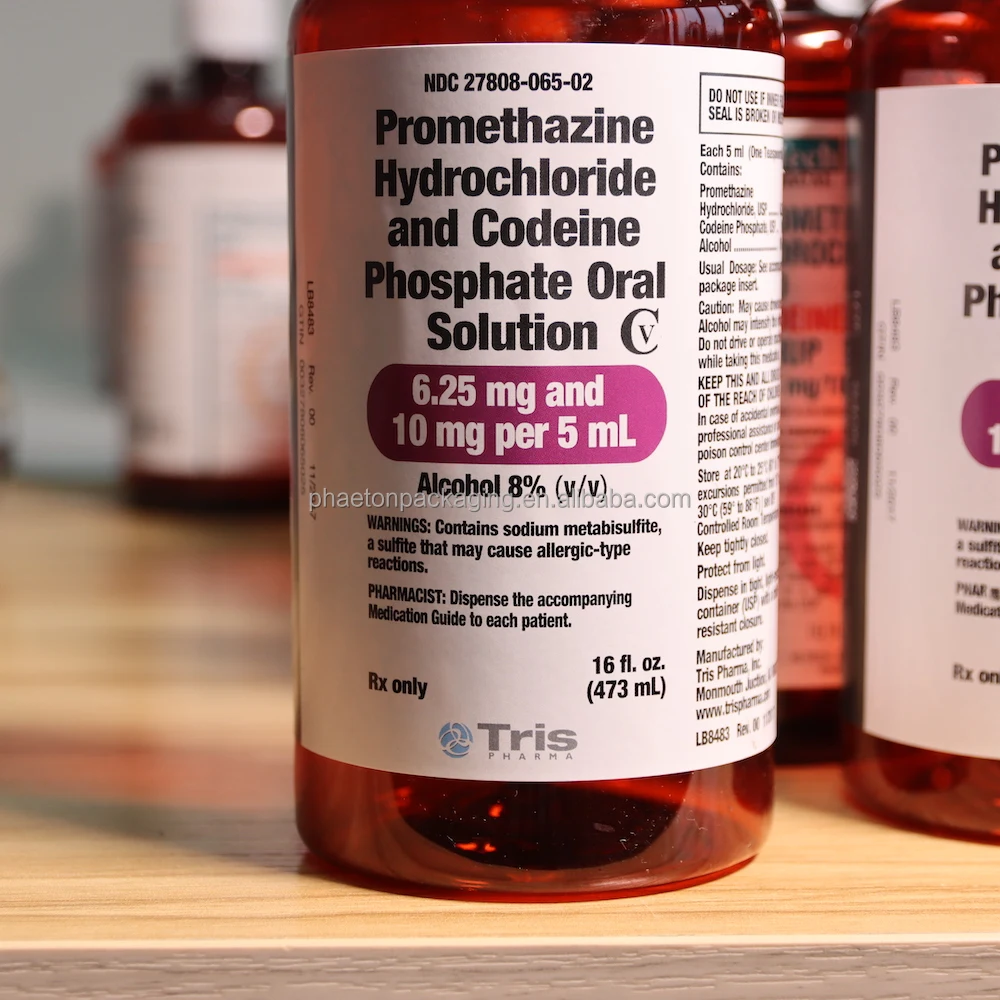
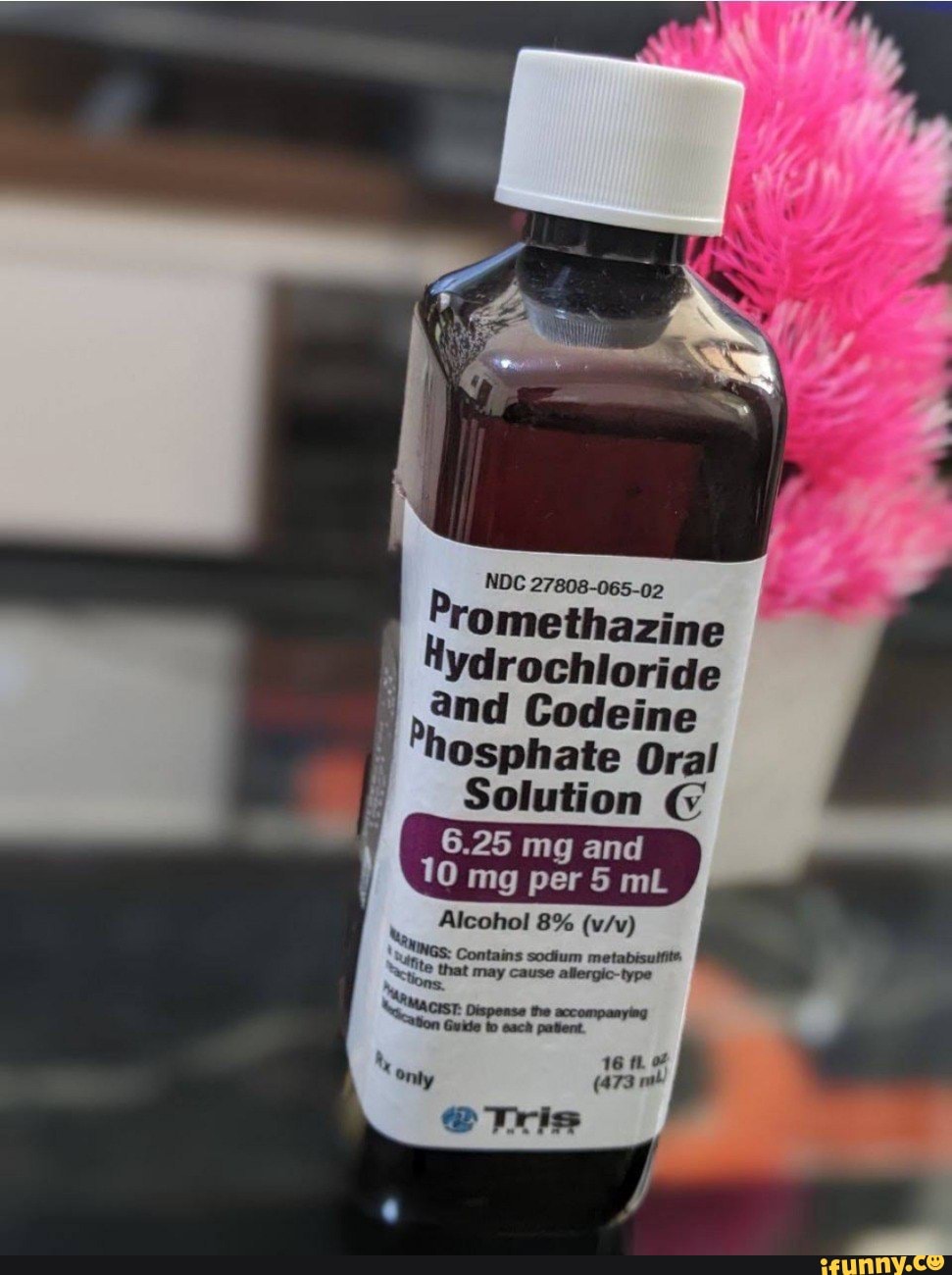
PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution - DailyMed NDC 27808-065-02 - Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV - 6.25 mg and 10 mg per 5 mL - Alcohol 8% (v/v) WARNINGS: Contains sodium metabisulfite, a sulfite that may cause ... INGREDIENTS AND APPEARANCE Product Information View All Sections Find additional resources (also available in the left menu) Safety

Ndc 27808-065-02
DailyMed - Search Results for CODEINE PHOSPHATE NDC Code (s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc PROMETHAZINE HYDROCHLORIDE, PHENYLEPHRINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution ... view full title NDC Code (s): 50383-805-04, 50383-805-16 Packager: Akorn PSEUDODINE C (triprolidine hydrochloride, pseudoephedrine hydrochloride and codeine phosphate) syrup ... 27808-085 Ndc - Dextroamphetamine PRODUCT NDC: 27808-085: The labeler code and product code segments of the National Drug Code number, separated by a hyphen. Asterisks are no longer used or included within the product code segment to indicate certain configurations of the NDC. PRODUCT TYPE NAME: HUMAN PRESCRIPTION DRUG: NDC 27808-051-02 Promethazine Hydrochloride Syrup Oral The NDC Packaged Code 27808-051-02 is assigned to a package of 473 ml in 1 bottle of Promethazine Hydrochloride, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is syrup and is administered via oral form. Is NDC 27808-051 included in the NDC Directory?
Ndc 27808-065-02. NDC 27808-065 Promethazine Hydrochloride And Codeine Phosphate The NDC code 27808-065 is assigned by the FDA to the product Promethazine Hydrochloride And Codeine Phosphate which is a human prescription drug product labeled by Tris Pharma Inc. The product's dosage form is solution and is administered via oral form. DailyMed - Search Results for CODEINE NDC Code(s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc; PROMETHAZINE HYDROCHLORIDE, PHENYLEPHRINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution. NDC Code(s): 50383-805-04, 50383-805 ... Promethazine and Codeine: Package Insert - Drugs.com Promethazine and Codeine Description. Promethazine with Codeine Oral Solution contains codeine an opioid agonist, and promethazine, a phenothiazine. Each 5 mL of Promethazine with Codeine Oral Solution contains 10 mg of codeine phosphate and 6.25 mg of promethazine hydrochloride for oral administration. NDC 27808-065-02 Promethazine Hydrochloride And Codeine Phosphate The NDC Code 27808-065-02 is assigned to a package of 473 ml in 1 bottle, plastic of Promethazine Hydrochloride And Codeine Phosphate, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is solution and is administered via oral form.
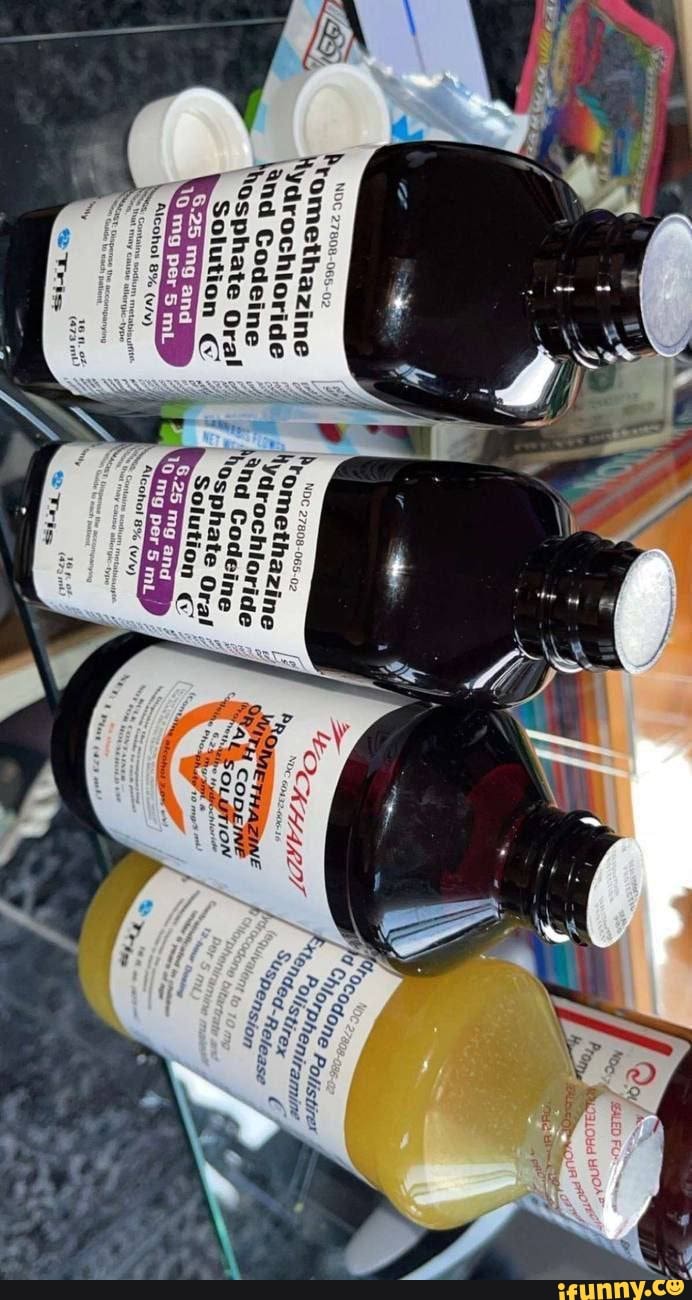
View our high-quality generics | Tris Pharma The Tris Pharma Generics division is focused on creating high-quality, patient-friendly products that leverage our strengths in product selection, product development, commercial launch, and securing market share. Driven by innovation, we are working to bring to market 20+ generic products in our pipeline and transform new ideas into effective ... NDC 27808-035-01 Hydrocodone Bitartrate And Acetaminophen The NDC Packaged Code 27808-035-01 is assigned to a package of 100 tablet in 1 bottle, plastic of Hydrocodone Bitartrate And Acetaminophen, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is tablet and is administered via oral form. Is NDC 27808-035 included in the NDC Directory? DailyMed - Search Results for promethazine NDC Code (s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHOPHAN HYDROBROMIDE solution NDC Code (s): 50383-803-04, 50383-803-08, 50383-803-16 Packager: Akorn PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE ORAL SOLUTION solution NDC Code (s): 70436-155-41, 70436-155-42 SEARCH RESULTS for: PROMETHAZINE HYDROCHLORIDE AND CODEINE ... - DailyMed PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE syrup. NDC Code (s): 17856-0804-5. Packager: Atlantic Biologicals Corps. This is a repackaged label. Source NDC Code (s): 50383-804. PROMETHAZINE VC WITH CODEINE ORAL SOLUTION (promethazine and phenylephrine hydrochloride and codeine phosphate) solution.
2019 ASP Drug Pricing Files | CMS 2019 ASP Drug Pricing Files. The Medicare Part B Drug and Biological Average Sales Price Quarterly Payment files for calendar year 2019 are located in the "Downloads" section below. Page Last Modified: 12/01/2021 08:00 PM. Help with File Formats and Plug-Ins. NDC 27808-051-02 Promethazine Hydrochloride Syrup Oral The NDC Packaged Code 27808-051-02 is assigned to a package of 473 ml in 1 bottle of Promethazine Hydrochloride, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is syrup and is administered via oral form. Is NDC 27808-051 included in the NDC Directory? 27808-085 Ndc - Dextroamphetamine PRODUCT NDC: 27808-085: The labeler code and product code segments of the National Drug Code number, separated by a hyphen. Asterisks are no longer used or included within the product code segment to indicate certain configurations of the NDC. PRODUCT TYPE NAME: HUMAN PRESCRIPTION DRUG: DailyMed - Search Results for CODEINE PHOSPHATE NDC Code (s): 27808-065-01, 27808-065-02 Packager: Tris Pharma Inc PROMETHAZINE HYDROCHLORIDE, PHENYLEPHRINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution ... view full title NDC Code (s): 50383-805-04, 50383-805-16 Packager: Akorn PSEUDODINE C (triprolidine hydrochloride, pseudoephedrine hydrochloride and codeine phosphate) syrup ...
Stiker Label Perekat Vinil Tahan Air Sirup Tris Baru 2021new - Buy Perekat Label Stiker,Sirup Obat Batuk Perekat Vinyl Labe,Tris Sirup Obat Batuk ...
![Stream Dunce [Lyrics N Description] by WiseMcguyver | Listen ...](https://i1.sndcdn.com/artworks-LzhzhGeAif9PzO7n-kMcKJg-t500x500.jpg)












![2780 COL. promet HARD] 60432-606-16 PRO. JETH, cop INE ORAL ...](https://img.ifunny.co/images/9b4a83cca9e3968f1179835a7978638526ea2924f636830970775827283d1cc7_1.jpg)

![Stream DJ WKILLA | Listen to BEAT Á VENDA [Exclusivos] 2023 ...](https://i1.sndcdn.com/artworks-XEZofLgVFbRAPTDp-BsyxWQ-t500x500.jpg)














Post a Comment for "39 ndc 27808-065-02"