44 label each reactant and product in the given chemical reaction
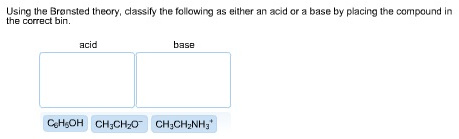
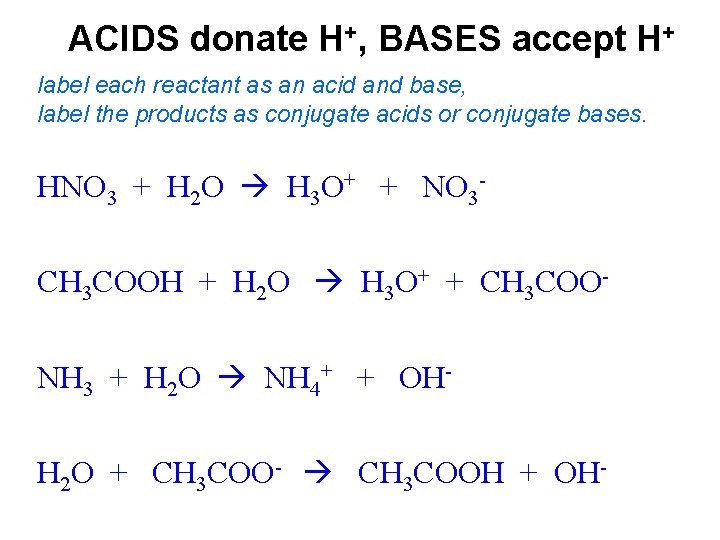
Answered: 1 Label each reactant and product in… | bartleby Q: Label each reactant and product in this reaction as a Brønsted acid or base. CH3OH+OH−↽−−⇀CH3O−+H2O. A: Interpretation: The given each reactant and product of the reaction are to be labelled as a Bronsted… Answered: For the reaction scheme below, label… | bartleby Transcribed Image Text: For the reaction scheme below, label each reactant as either a Lewis acid/base (LA or LB) or a Bronsted-Lowry acid/base (BLA or BLB). Use the boxes directly above each reactant for your answer. You only need to write LA or LB or BLA or BLB for your answer. Then, answer the questions below regarding this reaction.
5.3: Types of Chemical Reactions - Chemistry LibreTexts

Label each reactant and product in the given chemical reaction
Organic Chemistry Q&A.docx - 1. Label each reactant... 1. Label each reactant according to its role (or roles) in the chemical reaction. Check all that apply. 2. Add curved arrows to the reactant side of the SN2SN2 reaction shown. 3. Select the properties of the SN2SN2 reaction mechanism. 4. Draw the major organic product of the reaction. Answered: Label the following parts of the… | bartleby To check if an equation is balanced or not, simply count the number of atoms of each element on the reactant side of the equation and check to see if there are the same number of atoms of each or the same number and kind of element on the side of the equation. If so, it is balanced. Chemical reactions | Chemistry of life | Biology (article) - Khan Academy
Label each reactant and product in the given chemical reaction. 8.2 How do we represent chemical reactions? - Siyavula 1. Word equations. In mathematic equations we use an equal sign (=) for example 2 + 2 = 4, but in scientific chemical equations, we use an arrow (→), for example C + O 2 → CO 2. When we represent a chemical reaction in terms of words, we write a word equation. For example, when hydrogen gas reacts with oxygen gas to form water, we can write ... How would you label each formula in the chemical equation below as ... Explanation: The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, F e +S → F eS Iron and sulfur are the reactants, and iron sulfide is the product. Answer link Worked example: Calculating amounts of reactants and products A balanced chemical equation shows us the numerical relationships between each of the species involved in the chemical change. Using these numerical relationships (called mole ratios), we can convert between amounts of reactants and products for a given chemical reaction. Chapter 5: Chemical Reactions Flashcards | Quizlet Study with Quizlet and memorize flashcards terms like WHen the coefficient "4" precedes the reactant NaClO3 in a balanced chemical equation, there are _____ O atoms. - 3 - 4 - 12 - 20, Place the following calculations in the correct order to determine the formula weight of H2SO4. - Formula weight of H2SO4 = 98.09 g/mol - Formula weight of H2SO4 = (2 x M of H) + (M of S) + (4 x M of O ...
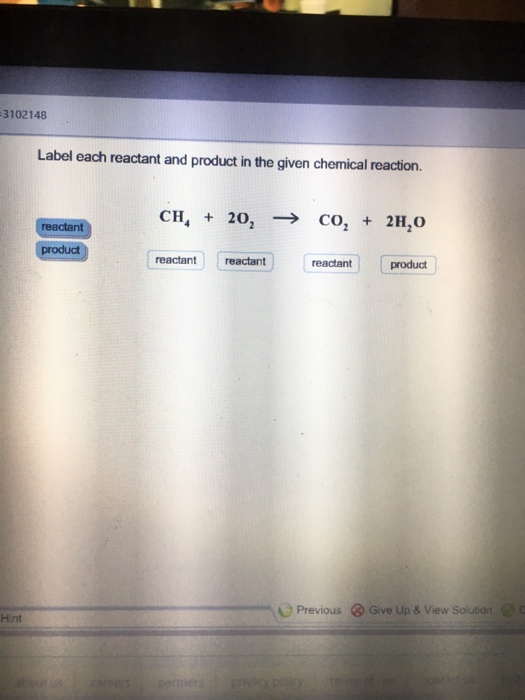
Answered: Label each reactant as either a… | bartleby Solution for Label each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. a. HF(aq) + H2O(l) ... −+CH3CH2OH Classify each reactant and product as an acid or base according… A: Given , a chemical reaction . CH3CH2O-+CH≡CH⇌HC≡C-+CH3CH2OHFor the above reaction , we… Solved Label each reactant and product in the given chemical | Chegg.com Expert Answer 89% (28 ratings) CH4=REACTANT O2 = REACT … View the full answer Transcribed image text: Label each reactant and product in the given chemical reaction. CH_4 + 2O_2 rightarrow CO_2 + 2H_2O Previous question Next question Answered: Classify each reactant and product in… | bartleby Homework help starts here! ASK AN EXPERT. Science Chemistry Q&A Library Classify each reactant and product in this reaction as an acid or base according to the Brønsted theory. HF+H2O↽−−⇀F−+H3O+. Classify each reactant and product in this reaction as an acid or base according to the Brønsted theory. HF+H2O↽−−⇀F−+H3O+. Chemistry chapter 8 & 9 Flashcards | Quizlet If one knows the mole ratio of a reactant and product in a chemical reaction, one can. calculate the mass of the product produced from a known mass of reactant. Given the equation 3A + 2B --> 2C, the starting mass of A, and its molar mass, and you are asked to determine the moles of C produced, your first step in solving the problem is the ...
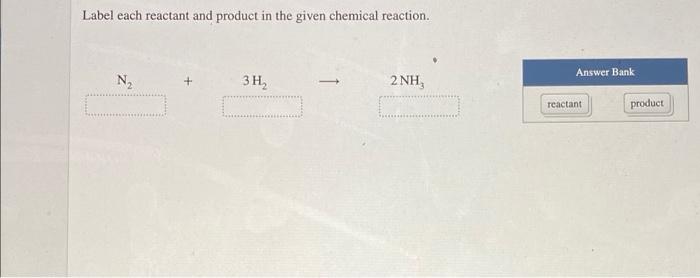
) In the reaction below, label each reactant as a nucleophile or an ... Answered step-by-step ) In the reaction below, label each reactant as a nucleophile or an electrophile. CH3COO- + O2S (OCH3)2 → CH3COOCH3 + CH3SO4- Chemistry Science Organic chemistry Answer & Explanation Solved by verified expert All tutors are evaluated by Course Hero as an expert in their subject area. CH3COO- ................> nucleophile Solved Label each reactant and product in the given chemical | Chegg.com Question: Label each reactant and product in the given chemical reaction. Nt 3H, 2 NH Automobile airbags inflate due to the formation of nitrogen gas from the chemical reaction 2 NaN, (s) + 3N, (B) + 2 Na (s) Identify the number of each atom in the reactants and products for this balanced reaction. (Solved) - Label each reactant and product in the given chemical ... Label each reactant and product ... Reactants and Products in Chemical Reactions - dummies They indicate the number of each chemical species that reacts or is formed. Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible.
Reactants and Products - GeeksforGeeks The reactants in the formation of water are hydrogen (H2) and oxygen (O 2) gas. Water is the product (H 2 O). Carbon dioxide (CO 2) and water are the reactants in photosynthesis (H 2 O). Glucose is the product (C 6 H 12 O 6 ). It should be noted that sunlight is not a reactant. Reactants are matter (atoms, molecules, and ions) rather than energy.
K2 - jejkz.rc-world.nl 2022. 7. 26. · Whether you purchase our in-house product line, need a private label, or want a custom blend, you'll get personalized service and high-quality formulations from Arrow. ... which relates reaction rate to temperature.A broad generalization of the Arrhenius equation is to say the reaction rate for many chemical reactions doubles ...
SOLVED:Label each reactant as a Brønsted-Lowry acid or a ... - Numerade Now with bronze did Lowry acid based definitions. Then we end up creating a conjugate acid and conjugate base. On the product side, the acid produces a conjugal base and the base produces a conjugate acid. In the next chemical reaction, we see that C H three n h two is accepting the hydrogen ion.
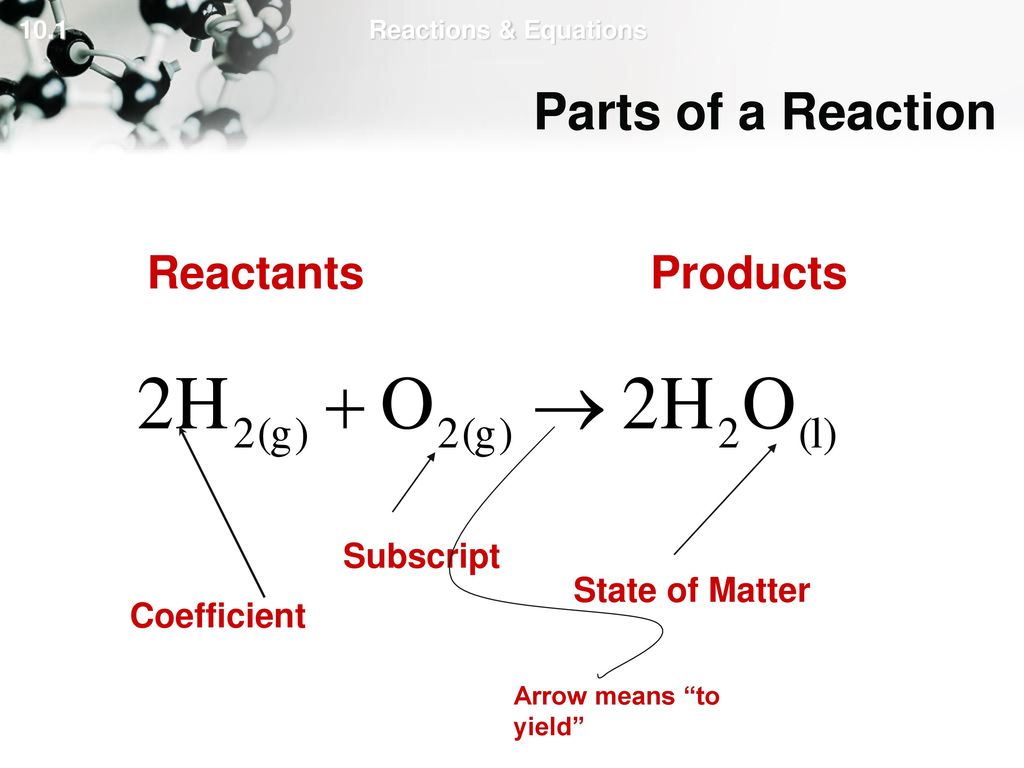
Chemical Reactions - Purdue University Chemical reactions are described by chemical equations. Example: The reaction between hydrogen and oxygen to form water is represented by the following equation. 2 H 2 + O 2 2 H 2 O. It is often useful to indicate whether the reactants or products are solids, liquids, or gases by writing an s, l , or g in parentheses after the symbol for the ...
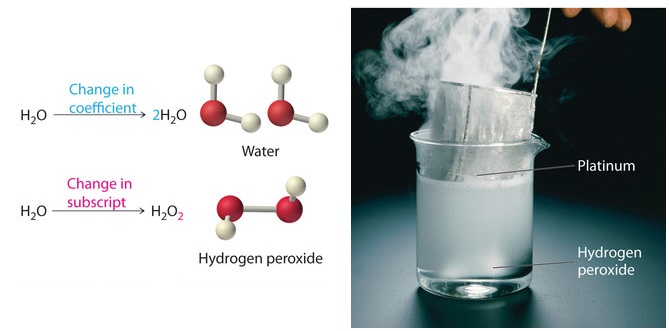
CH104: Chapter 5 - Chemical Reactions - Chemistry This should be the first consideration when writing a chemical reaction.The second consideration should be the states of the matter involved in the chemical reaction. Label each as a solid (s), a liquid (ℓ), a gas (g), or an aqueous solution (aq).
2.17: Reactants and Products - Chemistry LibreTexts
Lab Report Types of Reactions.pdf - Neel Raje Ms. Missig... Reaction 4: Heat Sodium Carbonate Ø Use a small spatula to place about half of an inch of sodium carbonate in a clean, dry test tube. Ø Record the appearance (colors, texture) of the reactant in the data table. Ø Label each reactant type. Then, choose the type of reaction you expect for the reactants.
7.2 Reactants and products | Chemical reactions | Siyavula REACTANTS (before the reaction) → PRODUCTS (after the reaction) Do you see how the atoms have rearranged? This means a chemical reaction has taken place. Label the diagram with 'reactants' and 'product'. The reaction between carbon and oxygen takes place when we burn coal. Coal is carbon and when it burns in oxygen gas, carbon dioxide is formed.
Solved 1. Label each reactant and product in the given | Chegg.com See the answer 1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products.
Ch 10 Chemical Reactions Flashcards | Quizlet The number written in front of a reactant or product. Synthesis reaction. A chemical reaction in which two or more substances react to produce a single product. Combustion reaction. Oxygen combines with a substance and releases energy in the form of heat and light. Decomposition reaction.
reactants and products | Science Quiz - Quizizz Any substance that results from a chemical reaction is called a_____ reactants and products DRAFT. KG - 8th. 1 times. ... reactant(s): carbonic acid; product(s): water and carbon dioxide. Tags: Question 17 . SURVEY . 30 seconds . ... The mass will stay the same because the number of each kind of atom stays the same. Tags: Question 37 . SURVEY ...
Chemical Reactions and Equations - GitHub Pages Example 1. Write and balance the chemical equation for each given chemical reaction. Hydrogen and chlorine react to make HCl. Ethane, C 2 H 6, reacts with oxygen to make carbon dioxide and water.; Solution. Let us start by simply writing a chemical equation in terms of the formulas of the substances, remembering that both elemental hydrogen and chlorine are diatomic:
Chemical Reactions - Definition, Equations, Types, Examples with ...
Types of Chemical Reactions: How to classify five basic reaction types.
Label each reactant according to its role (or roles) in the chemical ... In a given reaction, the hydroxyl is acting as a Lewis base and the hydrogen bromide is acting as a Lewis acid giving the water and bromide as a product after reacting with each other. (OH-) Lewis base + (HBr) Lewis acid----> (HOH) + (Br-) Reaction no. 2 The following given reaction shows the nucleophilic and electrophilic attack reaction.
Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2
Overview of Water as a Reactant in the Chemical Reactions - GradesFixer
Chemical reactions | Chemistry of life | Biology (article) - Khan Academy
Answered: Label the following parts of the… | bartleby To check if an equation is balanced or not, simply count the number of atoms of each element on the reactant side of the equation and check to see if there are the same number of atoms of each or the same number and kind of element on the side of the equation. If so, it is balanced.
Organic Chemistry Q&A.docx - 1. Label each reactant... 1. Label each reactant according to its role (or roles) in the chemical reaction. Check all that apply. 2. Add curved arrows to the reactant side of the SN2SN2 reaction shown. 3. Select the properties of the SN2SN2 reaction mechanism. 4. Draw the major organic product of the reaction.






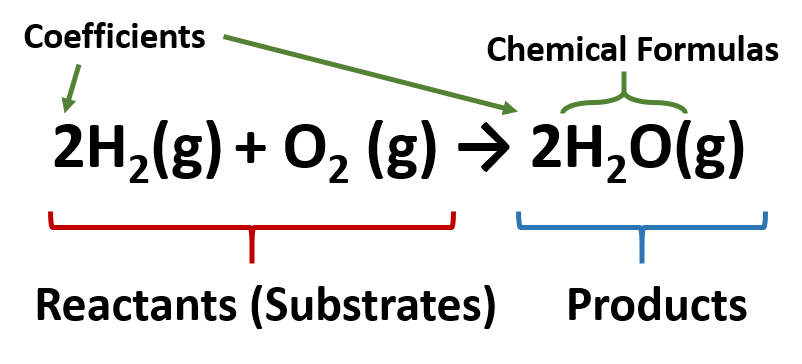





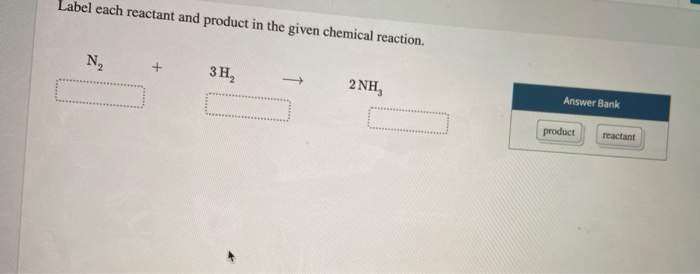




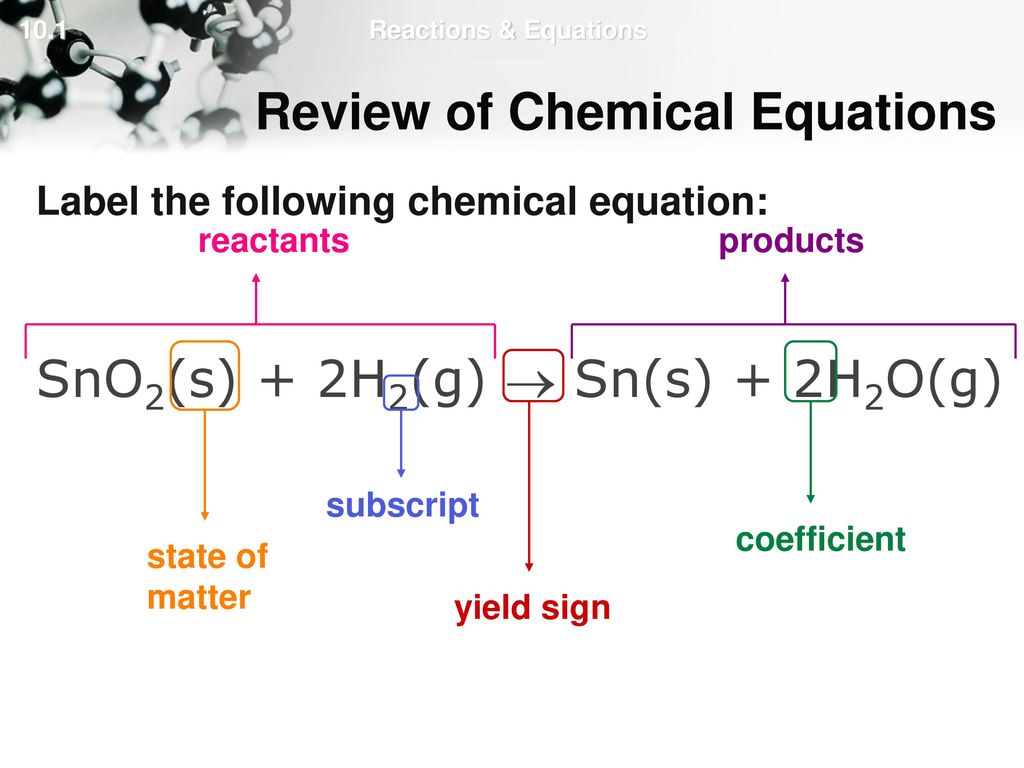




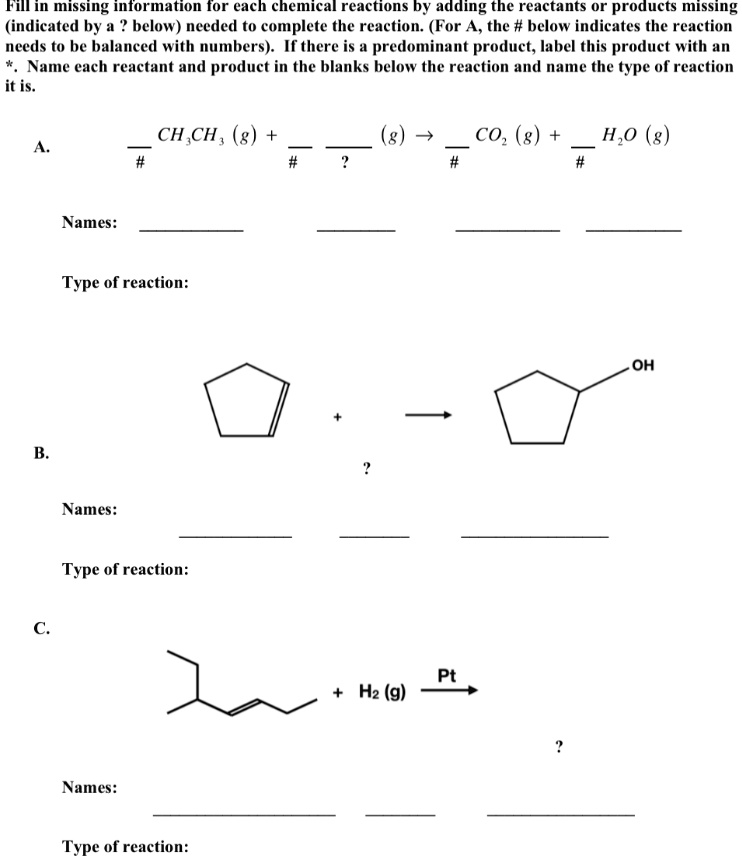






Post a Comment for "44 label each reactant and product in the given chemical reaction"