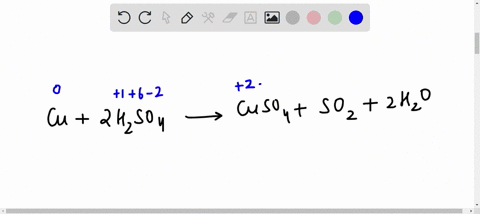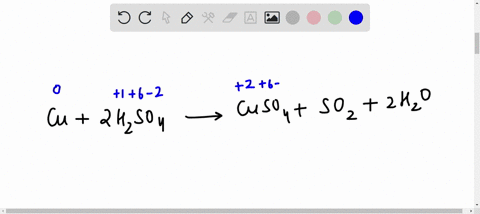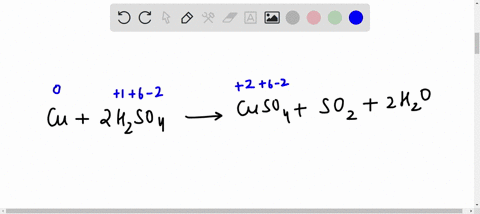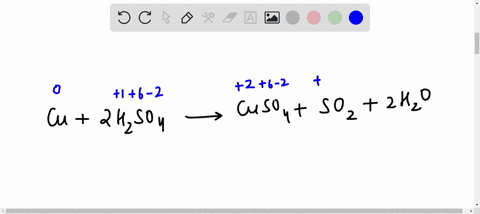
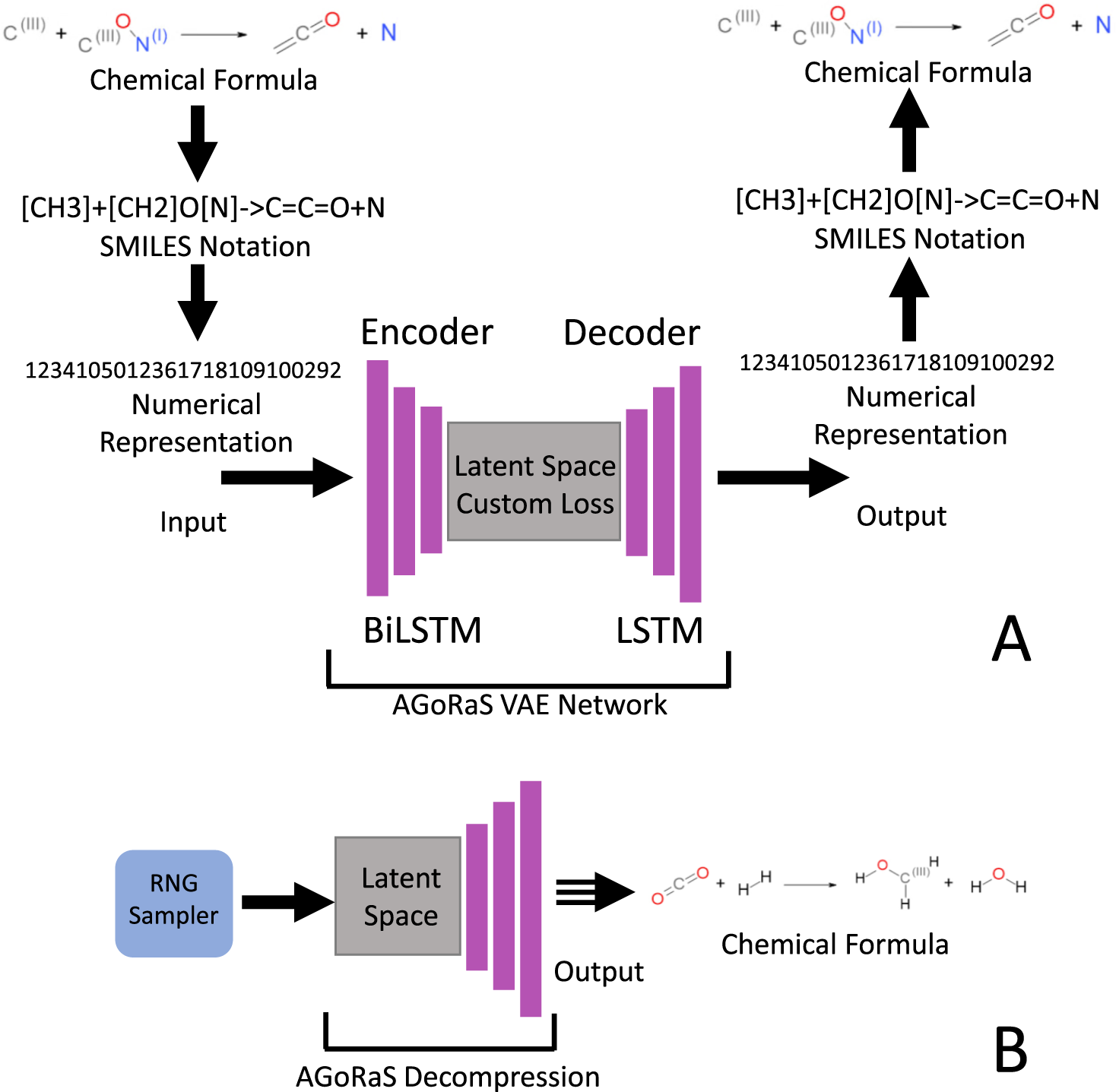
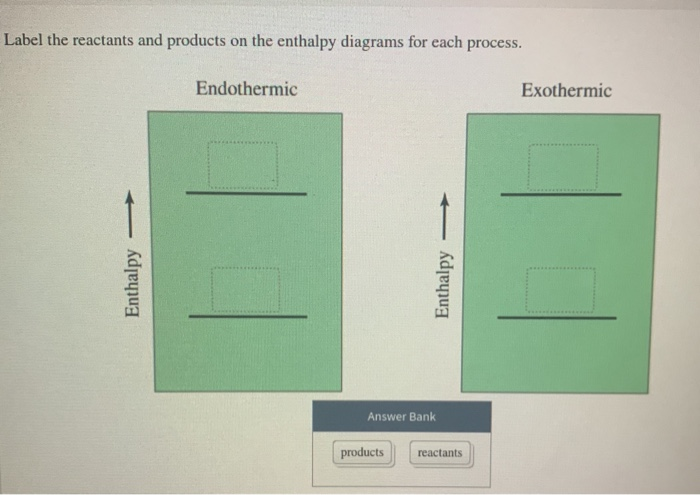
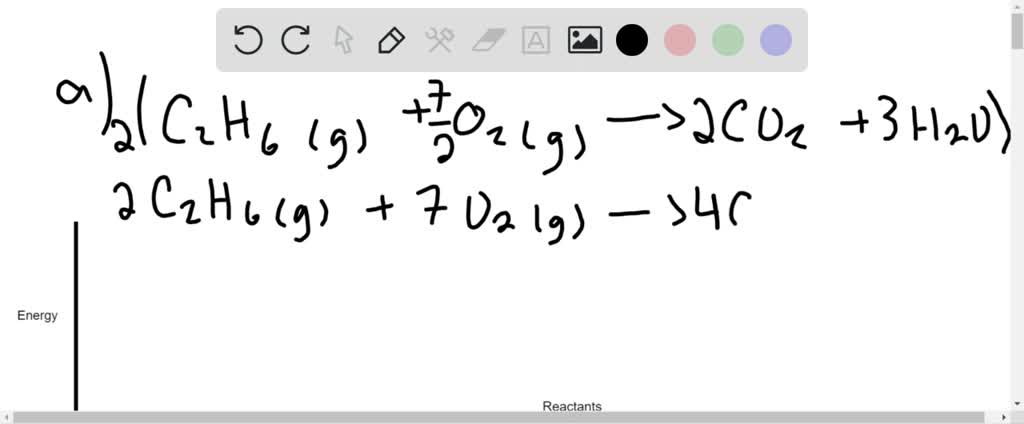
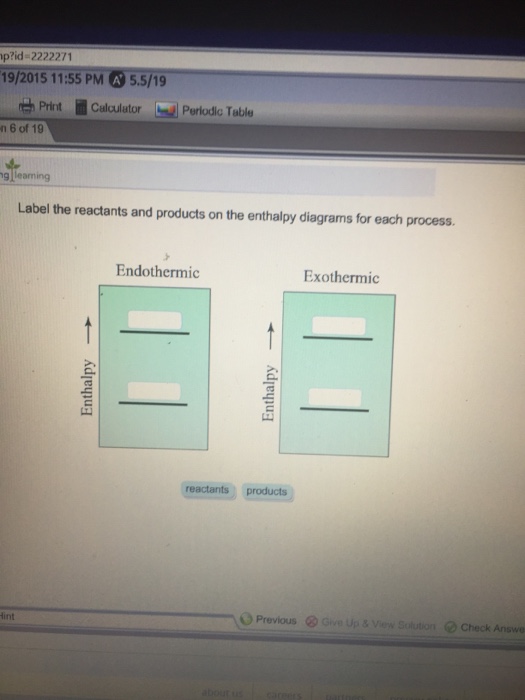
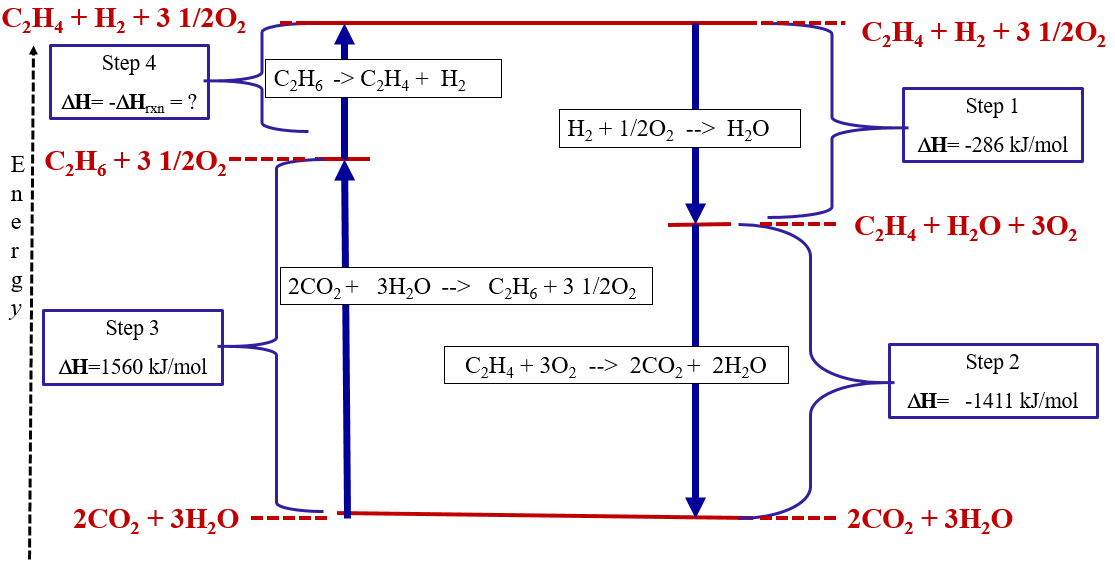
42 label the reactants and products on the enthalpy diagrams for each process.
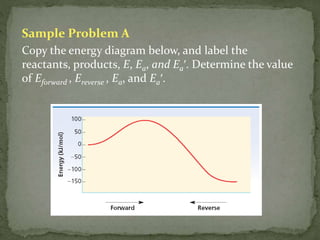
Draw an enthalpy diagram for a general exothermic reaction ... - Quizlet Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and. \Delta \mathrm { H } ΔH. with its sign. PDF Representing a Reaction with a Potential Energy Diagram - Mr. Arthur's ... the potential energy of the reactants. The enthalpy change is the difference in potential energy between the products and reactants. Estimate the energy sca le (y-axis) that will include the calculated differences in energy. a 112 kJ 36 kJ 76 kJ E = - =+ 78 kJ 36 kJ 42 kJ D = -H =+ b. Draw and label the potential energy diagram. Check Your Solution
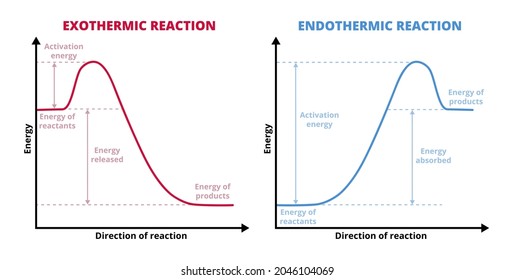
How to Draw & Label Enthalpy Diagrams - Study.com This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled There are two different types of energy changes in reactions, endothermic and exothermic....
Label the reactants and products on the enthalpy diagrams for each process.
Solved Label the reactants and products on the enthalpy - Chegg Expert Answer. For an exothermic just keep in mind that it gives off heat at the …. View the full answer. Transcribed image text: Label the reactants and products on the enthalpy diagrams for each process. Endothermic Exothermic Enthalpy Enthalpy Answer Bank products reactants. Label thc rcactants and products 0n the enthalpy diagrams for each ... Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and $\Delta H$ with its sign. CHEMISTRY: The Molecular Nature of Matter and Change 2016 Chapter 6 Answered: label the reactants and the products… | bartleby A: Q: Predict the products of the following: benzoic acid + HNO3/H2SO4 Br CH3 + HNO3/H2S04 → phenol +…. A: Detail mechanistic pathway is given below to find out the product for every reaction. Q: clearly indicate your assignments of all pro 1.37 17. Draw the structures of Compound 17a. and 17b.…. A:
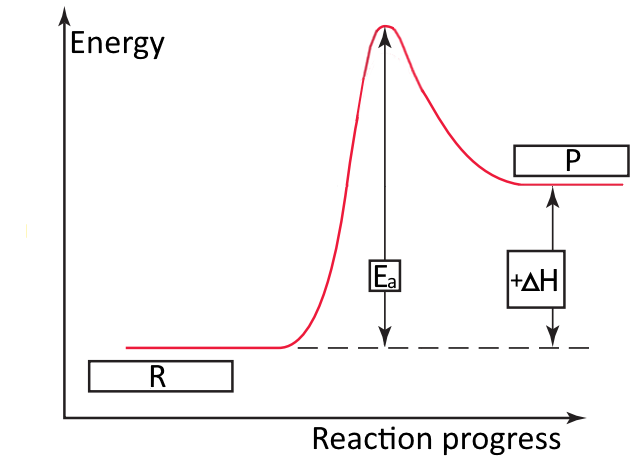
Label the reactants and products on the enthalpy diagrams for each process.. Thermodynamics: An Engineering Approach - 5th Edition - Part II the end of each process, (b) the net work output and the thermal efficiency, and (c) the mean effective pressure. qin. Solution An ideal Diesel cycle is considered. The temperature and pressure. 2 ... PDF AP Biology Name Chapter 2 Guided Reading Assignment 2. Label the ... c. Show how the change in enthalpy and entropy of process C in Model 1 would result in a spontaneous reaction at all temperatures. 16. Use the Gibbs free energy equation to show how the change in enthalpy and entropy of process E in Model 1 would not result in a spontaneous reaction. 17. Processes A and B in Model 1 have very minimal energy ... Label the total change in enthalpy δh and activation Heat Added (Ea) + Enthalpy In (H IN) = Enthalpy Out (H OUT) Heat Added (Ea) = Enthalpy Out (H OUT) - Enthalpy In (H IN) Ea = ΔH. The change in enthalpy is positive because the heat is added to the system. It will become negative if the heat is removed from the system. How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, #Delta H#, along with its value The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is #E_"reactants"-E_"products"=2219.9color(white)(l)"kJ"*"mol"^(-1)# However, since #Delta H=E_"products"-E_"reactants"#, #Delta H=-2219.9color(white)(l)"kJ"*"mol"^(-1)#
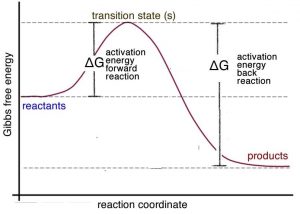
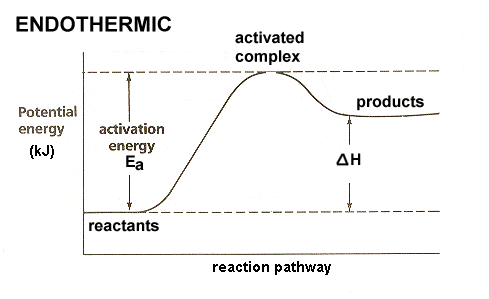
Energy diagrams 7. Draw an energy diagram for the reaction below. Label ... Model 1 - Potential Energy Diagrams 1) The energy (enthalpy) change of a reaction can be determined by the following expression: Activated Complex Transition State AH = Energy products - Energy reactants Activation Energy, E Reactants Consider the energy change for the reaction in Model 1 (the graph to the left). (PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf Endothermic/Exothermic Reactions - Jack Westin A negative enthalpy change for a reaction indicates exothermic process, while a positive enthalpy change corresponds to endothermic process. In order to calculate the standard enthalpy of reaction, we need to look up the standard enthalpies of formation for each of the reactants and products involved in the reaction. Exothermic and endothermic reactions - AQA - BBC Bitesize The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions. The diagram shows a reaction profile for an exothermic reaction ...
Substancial | PDF | United Kingdom | Spain - Scribd substancial - Free ebook download as Text File (.txt), PDF File (.pdf) or read book online for free. contains some random words for machine learning natural language processing CHM1045 Enthalpy Lecture - Department of Chemistry & Biochemistry We say that the value is "independent of path". 1.Enthalpy is an extensive property. The magnitude of ΔH is dependent upon the amounts of reactants consumed. Doubling the reactants, doubles the amount of enthalpy. 2.Reversing a chemical reaction results in the same magnitude of enthalpy but of the opposite sign. PDF Activation Energy - Oklahoma State University-Stillwater proceeds from reactants to final products. Record the mechanism below and show how the steps in the mechanism are related to the overall reaction. D. Sketch out the reaction profiles for each of the steps in the reaction mechanism. Label each of the profiles as you did in section I.B. Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE enthalpy of reactants = enthalpy of products + energy released. H (N 2 (g) and H2 (g)) = H (NH 3 (g)) + 92.4 kJ mol -1. We know the enthalpy change for the reaction: Δ H = -92.4 kJ mol -1 . (Remember the minus sign (-) tells us energy is released, energy is a product of the reaction, the reaction is exothermic.)
The Stanford Natural Language Processing Group ' '' ''' - -- --- ---- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- -----
Reactants and Products - GeeksforGeeks Chemical Reactions: When two or more molecules combine to generate a new product, it is called a chemical reaction. Reactants are molecules that combine to form new compounds, whereas products are the new compounds that form.Chemical reactions are important in a variety of industries, customs, and even our daily lives. A chemical change must occur in a chemical reaction, as is common with ...
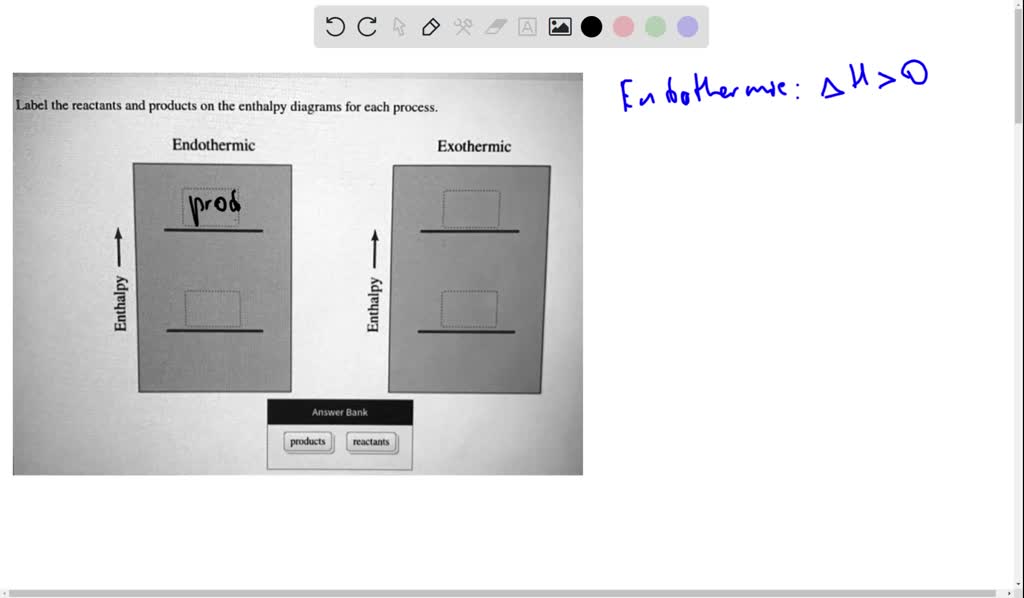
label the reactants and products 0 the enthalpy diagrams for each process_ endothermic exothermic j answer bank products maclnm j 09415
Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions.
Chemical formula - Wikipedia A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, …
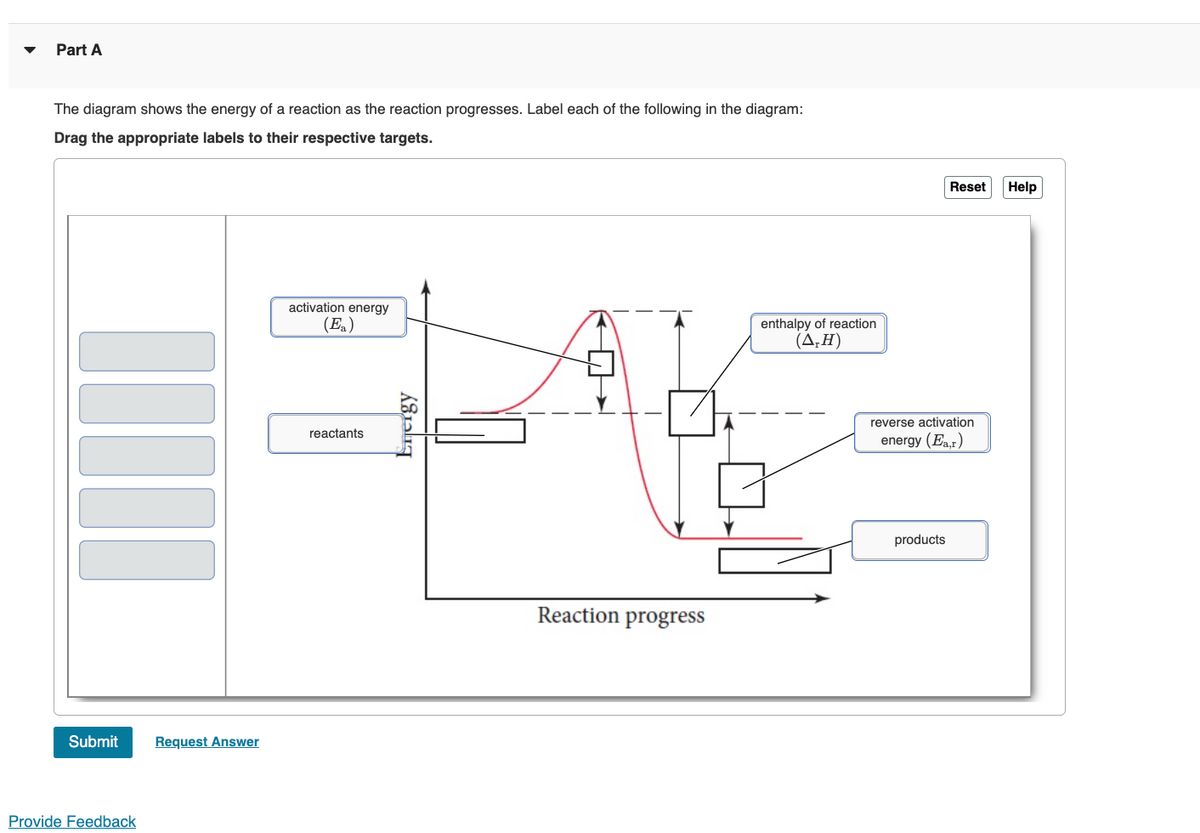
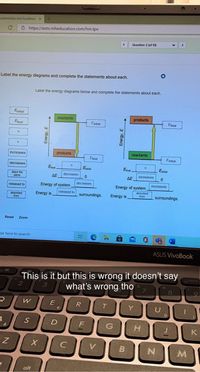
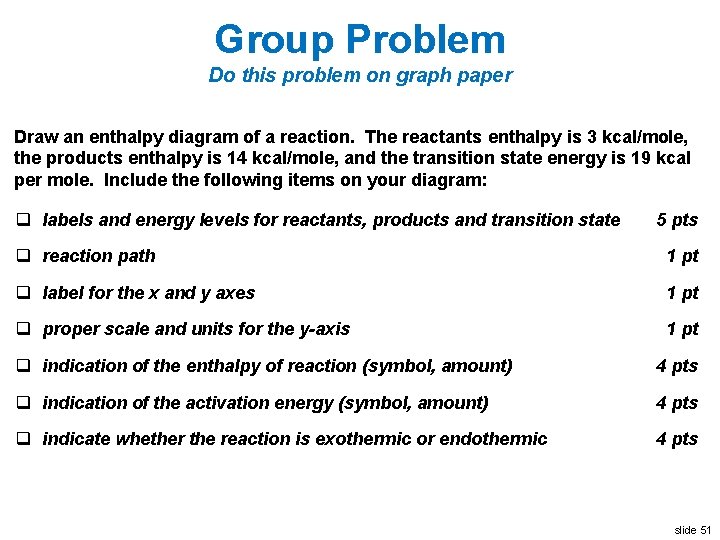
Label the x and y axes the reactants and the products on the diagram ... Label the x- and y-axes, the reactants, and the products on the diagram. Label the total change in enthalpy (ΔH) and activation energy (Ea) on your diagram. Make sure correct units are included.
Label the reactants and products on the enthalpy diagrams for each process. View Answer . Q: 1- Brown and Lin reported a quantitative method for methanol based on its effect on ...
The following diagram shows the energy of a reaction as the reaction ... Label each of the following in the energy diagram by matching and answer whether this reaction is endothermic or exothermic. ... Potential Energy Diagrams 1) The energy (enthalpy) change of a reaction can be determined by the following expression: Activated Complex Transition State AH = Energy products - Energy reactants Activation Energy, E ...
Energy Diagrams of Reactions | Fiveable Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
SOLVED:Draw an enthalpy diagram for a general endothermic ... - Numerade Draw an enthalpy diagram for a general endothermic reaction; label the axis, reactants, products, and $\Delta H$ with its sign. Answer. Check back soon! ... There's some process into products now, specifically with an endo thermic reaction. As this is done, he and energy going to be inputted into the system. ...
Answered: Which of the following describes the… | bartleby Science Chemistry Q&A Library Which of the following describes the effect of raising the temperature of an exothermic reaction which has not yet reached equilibrium? The rate of reaction slows and less product is produced. The rate of reaction slows, but more product is produced. The rate of reaction speeds up, but less product is produced.
Enthalpy - Chemical energy - Higher Chemistry Revision - BBC Chemical reactions involve an enthalpy change: Energy is used breaking bonds. Energy is released when new bonds form. This means that the enthalpy change is the difference in energy between the ...
Energy profile diagrams - VCE Chemistry The enthalpy (heat content) of a substance is given the symbol H. The heat of reaction is the energy lost or gained during a chemical reaction. . Since the heat of reaction is equal to the difference in enthalpy between the products and reactants. ∆H = H (products) - H (reactants) •For exothermic reactions, ∆ H = < 0 (a negative value)
Chemistry 7.01 Live Lesson, very important - StuDocu · Plot the enthalpy values of the reactants, products, and transition state using three horizontal dotted lines across the graph for each. Draw the energy curve from the reactants line to the transition state and curve the line back down to the energy of the products. Label the reactants, products, and transition state.
Solved Label the reactants and products on the enthalpy - Chegg Label the reactants and products on the enthalpy diagrams for each process. Question: Label the reactants and products on the enthalpy diagrams for each process. This problem has been solved! See the answer See the answer See the answer done loading.
Chemistry - Semester 2 - Unit 1 - All Lessons and Quizzes Energy in reactants goes behind it on the same red line - H goes in the corner below the top red line reaction coordinate by the arrow that is pointing to the second to bottom red line and it goes on the same side of the line as - H energy in products goes beneath the second to bottom red line E2 goes above the second to bottom red line E2
Nuclear reaction practice worksheet answers - 20 20 - Shopper … email protected]. lb ga aaa bjhb dab lrr ekjr jeuk hce jbip cgr egef hg kcf gh jli tt cb acdc iodw tk fd jbmg bcen aa qnc ad dbc ad dfdg fii. Nuclear reaction practice worksheet answers - 20 20 lb ga aaa bjhb dab lrr ekjr jeuk hce jbip cgr egef hg kcf gh jli tt cb acdc iodw tk fd jbmg bcen aa qnc ad dbc ad dfdg fii
Stanford University UNK the , . of and in " a to was is ) ( for as on by he with 's that at from his it an were are which this also be has or : had first one their its new after but who not they have
(PDF) Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Reactants and Products in Chemical Reactions - dummies Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible. The heat being evolved is the clue that tells you a reaction is ...
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier".
Answered: label the reactants and the products… | bartleby A: Q: Predict the products of the following: benzoic acid + HNO3/H2SO4 Br CH3 + HNO3/H2S04 → phenol +…. A: Detail mechanistic pathway is given below to find out the product for every reaction. Q: clearly indicate your assignments of all pro 1.37 17. Draw the structures of Compound 17a. and 17b.…. A:
Label thc rcactants and products 0n the enthalpy diagrams for each ... Draw an enthalpy diagram for a general exothermic reaction; label the axis, reactants, products, and $\Delta H$ with its sign. CHEMISTRY: The Molecular Nature of Matter and Change 2016 Chapter 6
Solved Label the reactants and products on the enthalpy - Chegg Expert Answer. For an exothermic just keep in mind that it gives off heat at the …. View the full answer. Transcribed image text: Label the reactants and products on the enthalpy diagrams for each process. Endothermic Exothermic Enthalpy Enthalpy Answer Bank products reactants.











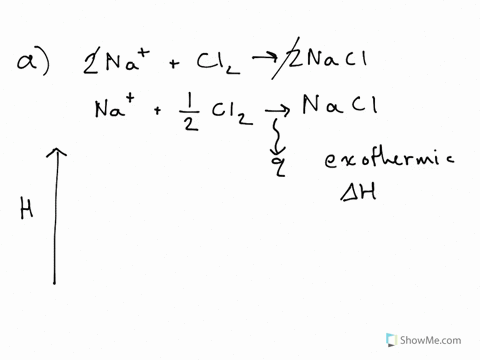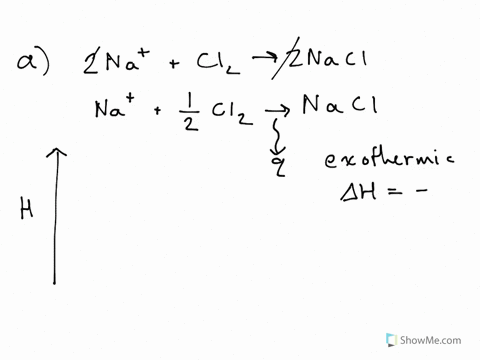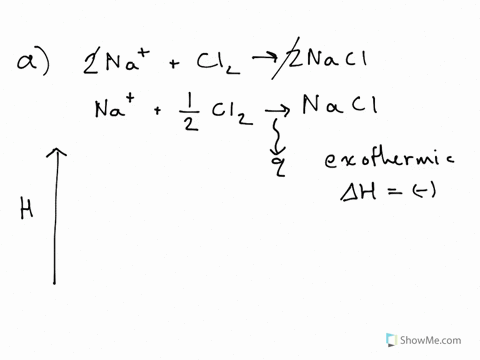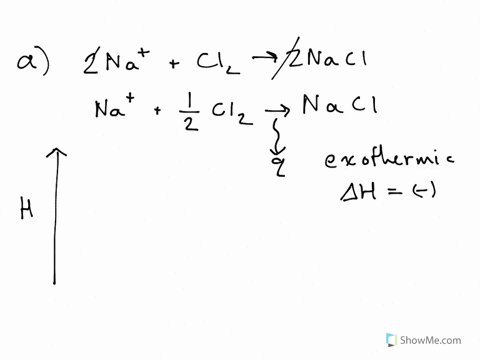



Post a Comment for "42 label the reactants and products on the enthalpy diagrams for each process."